Did you know that blood loss is the leading cause of preventable death after trauma, yet most ambulances don’t carry blood because it is perishable and type-specific?
Imagine if every first responder and hospital had shelf-stable blood stocked for use on any patient needing an emergency transfusion.
That’s why we are developing ErythroMer™
EXPLORE HOW IT WORKS:
TRANSFUSION
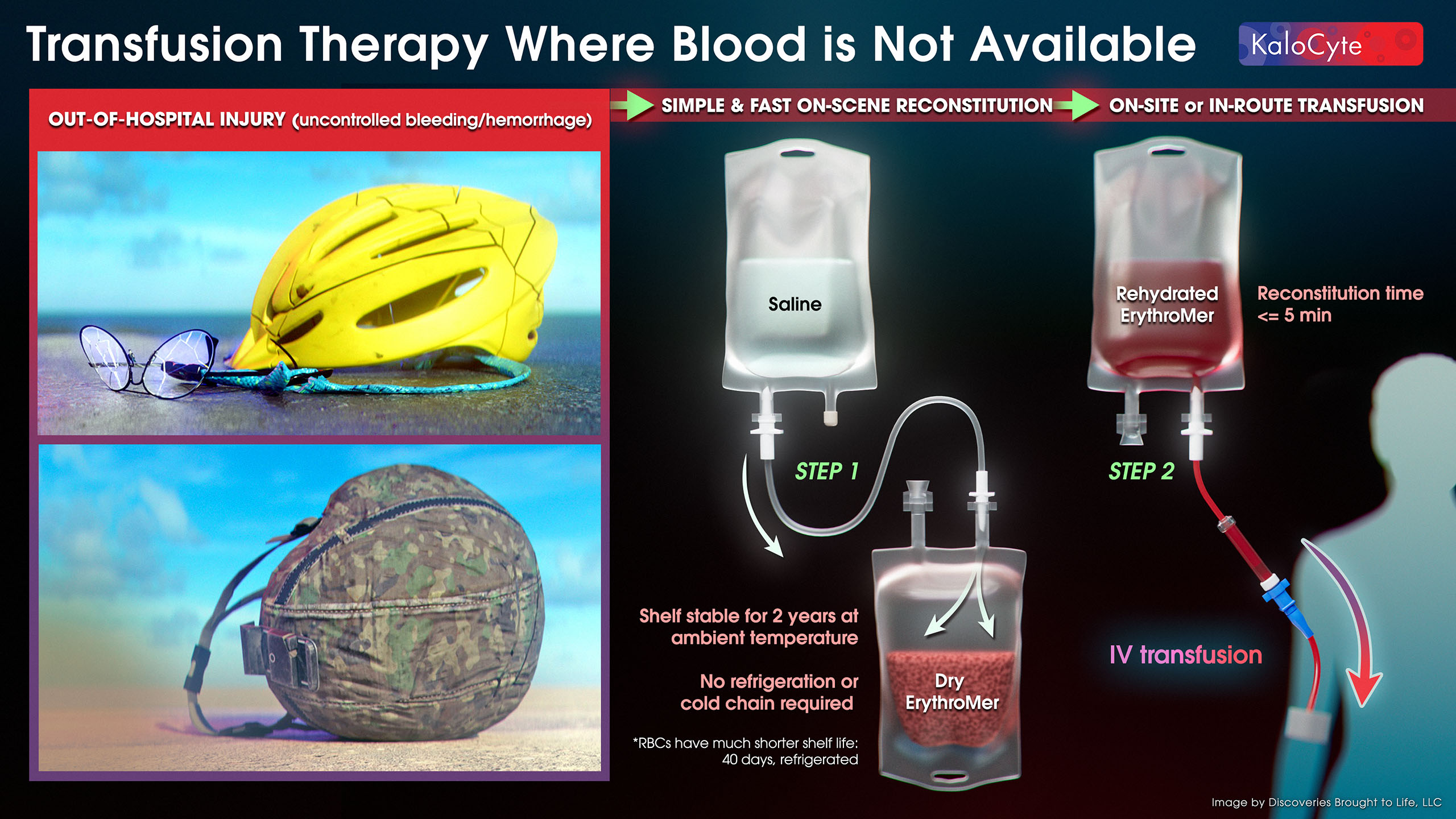
Blood loss after injury is responsible for over 25,000 lost lives each year in the US alone1, and billions of dollars in medical care and lost productivity. Every minute of delay in replacing lost blood increases mortality by 5%2, yet 47 million Americans live more than an hour from a trauma center3, and most ambulances do not carry blood. Once approved, ErythroMer™ will be supplied as a freeze-dried powder, shelf-stable without refrigeration, and packaged as a unit equivalent to one unit of red blood cells – “just add water” for rapid use at the point of care or on the way to the hospital.
1. Spinella, P.C. and A.P. Cap, Prehospital hemostatic resuscitation to achieve zero preventable deaths after traumatic injury. Curr Opin Hematol, 2017. 24(6): p. 529-535.
2. Meyer, D.E., et al., Every minute counts: Time to delivery of initial massive transfusion cooler and its impact on mortality. J Trauma Acute Care Surg, 2017. 83(1): p. 19-24.
3. Branas C.C., MacKenzie E.J., Williams J.C., Schwab C.W., Teter H.M., Flanigan M.C., ReVelle C.S. Access to trauma centers in the United States. JAMA. 2005;293:2626–2633. doi: 10.1001/jama.293.21.2626.
MOLECULAR ARCHITECTURE
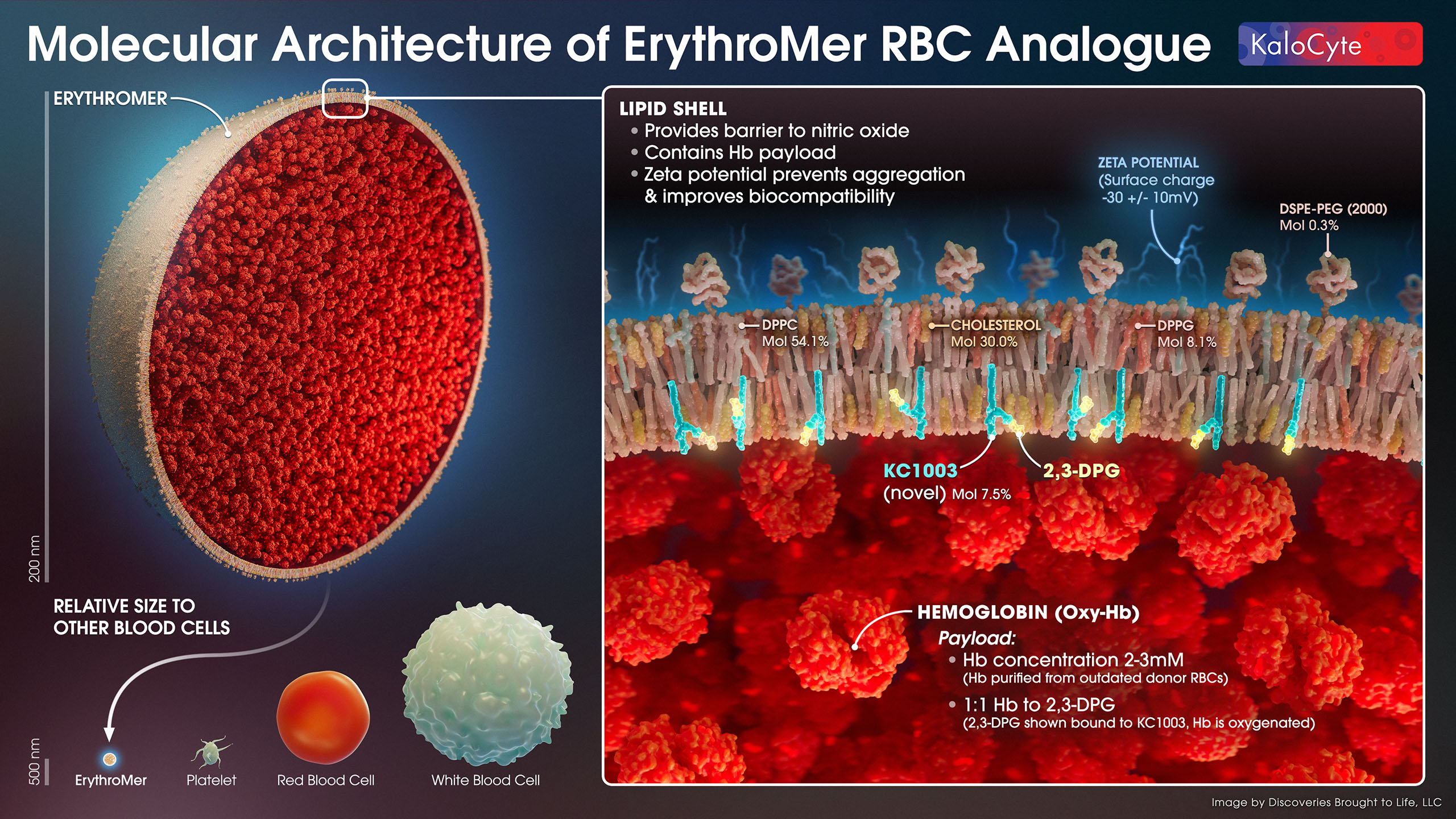
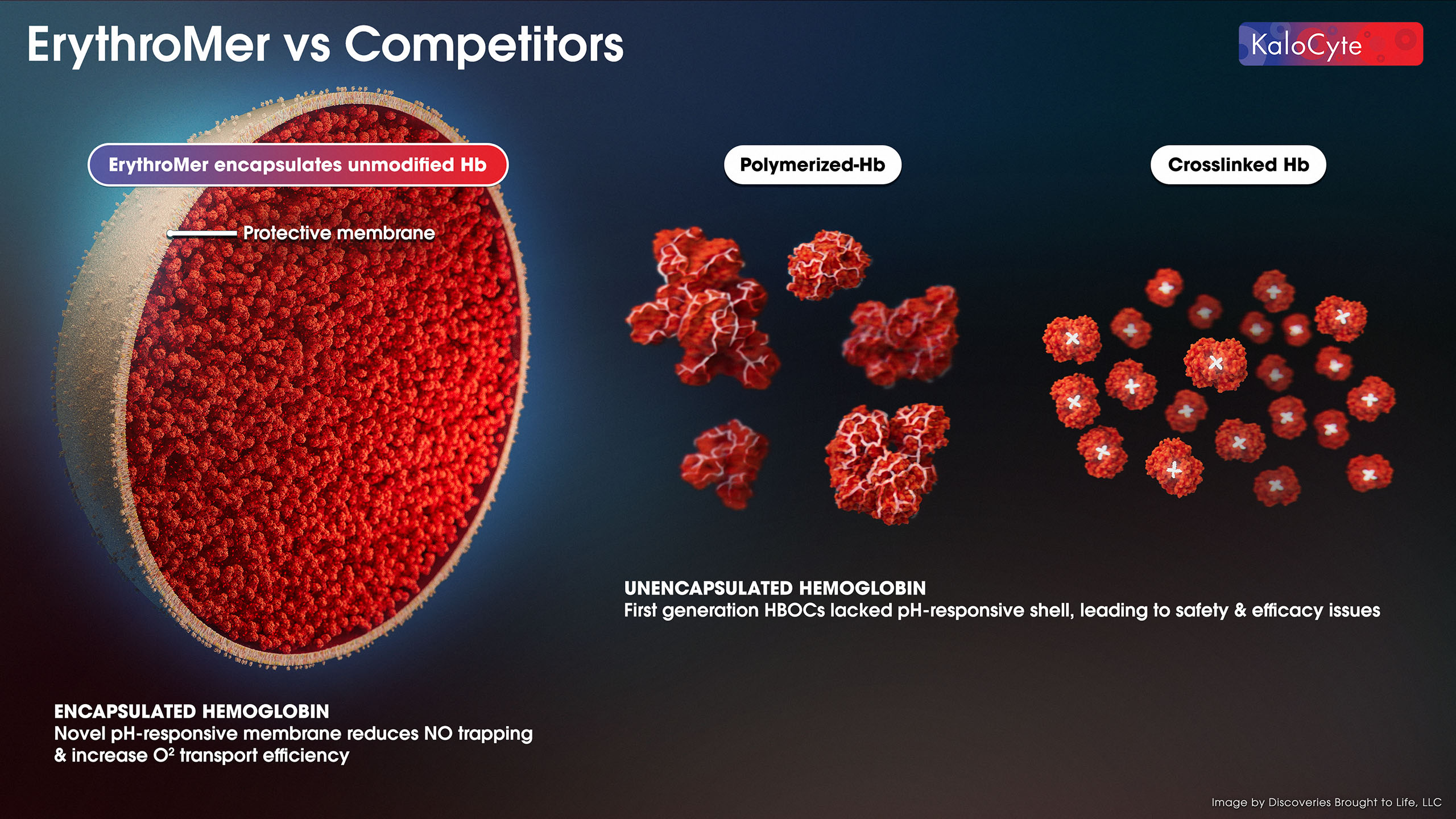
ErythroMer™ encases purified human hemoglobin (Hb) in a soft lipid nanoparticle “shell” designed to mimic native RBCs, and is a “universal option” for all blood types. The lipid shell provides a barrier to nitric oxide, contains the Hb payload, and has a surface charge (zeta potential) that prevents aggregation and improves biocompatibility.
KC1003, our novel and proprietary molecule, regulates 2,3-DPG binding to Hb by capturing it at high pH (lungs) and releasing it at low pH (peripheral tissues). Here we show a high pH state: ErythroMer’s payload is packed with oxygenated Hb and KC1003 is bound to 2,3-DPG. See Oxygen Delivery.
OXYGEN DELIVERY
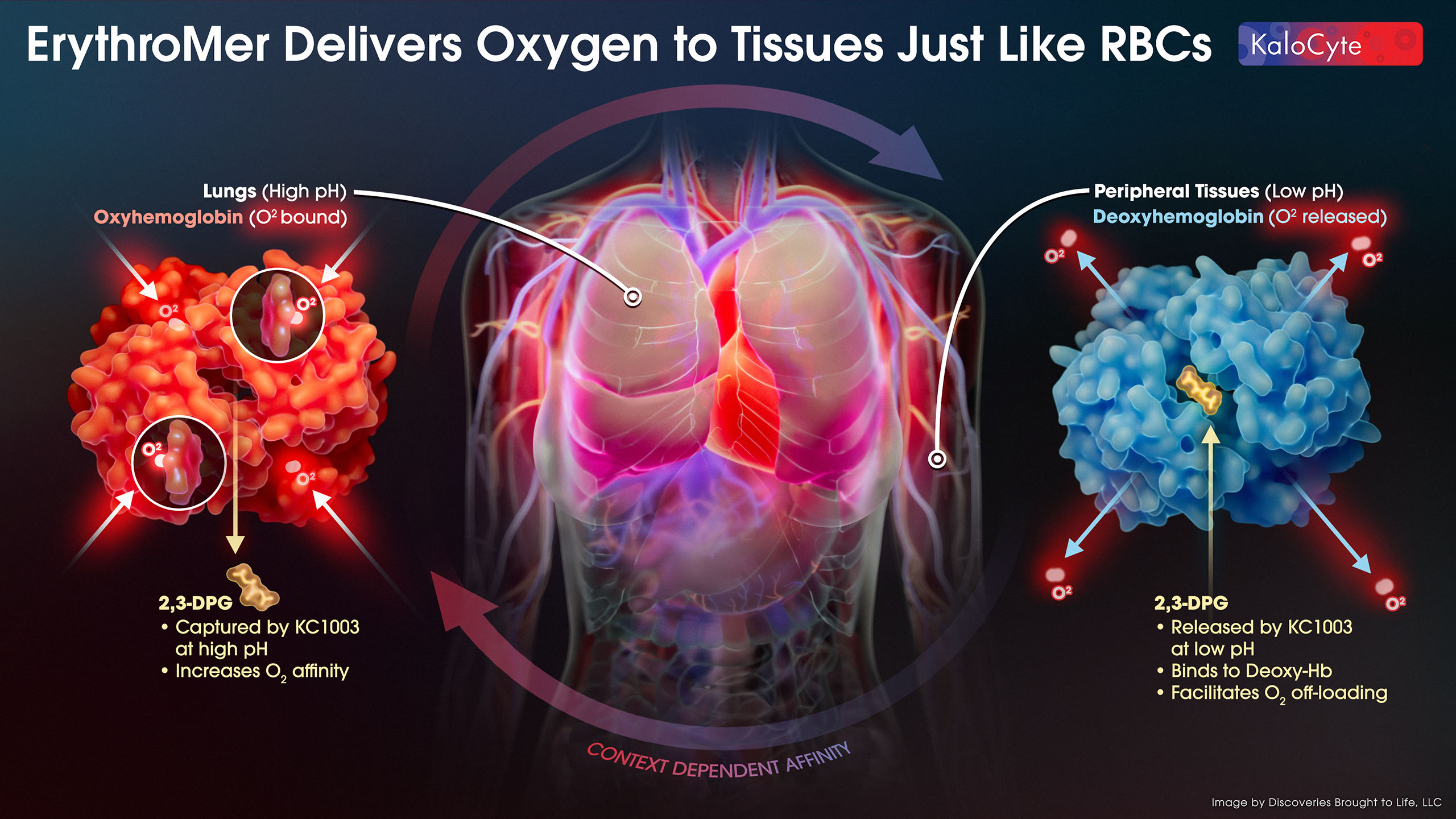
ErythroMer™ is specifically designed to 1) mimic natural RBC physiology and 2) address the flaws of prior Hemoglobin Based Oxygen Carriers (HBOCs, see Competition). Its small size and bioinspired design make it a highly efficient oxygen carrier to pick up oxygen in the lungs and deliver it to hypoxic tissues throughout the body. KC1003 (not shown here, see Molecular Architecture), regulates 2,3-DPG binding to Hb by capturing it at high pH and releasing it at low pH. Regulation of 2,3-DPG by KC1003 ensures oxygen is quickly delivered to tissues that need it most.
METABOLISM
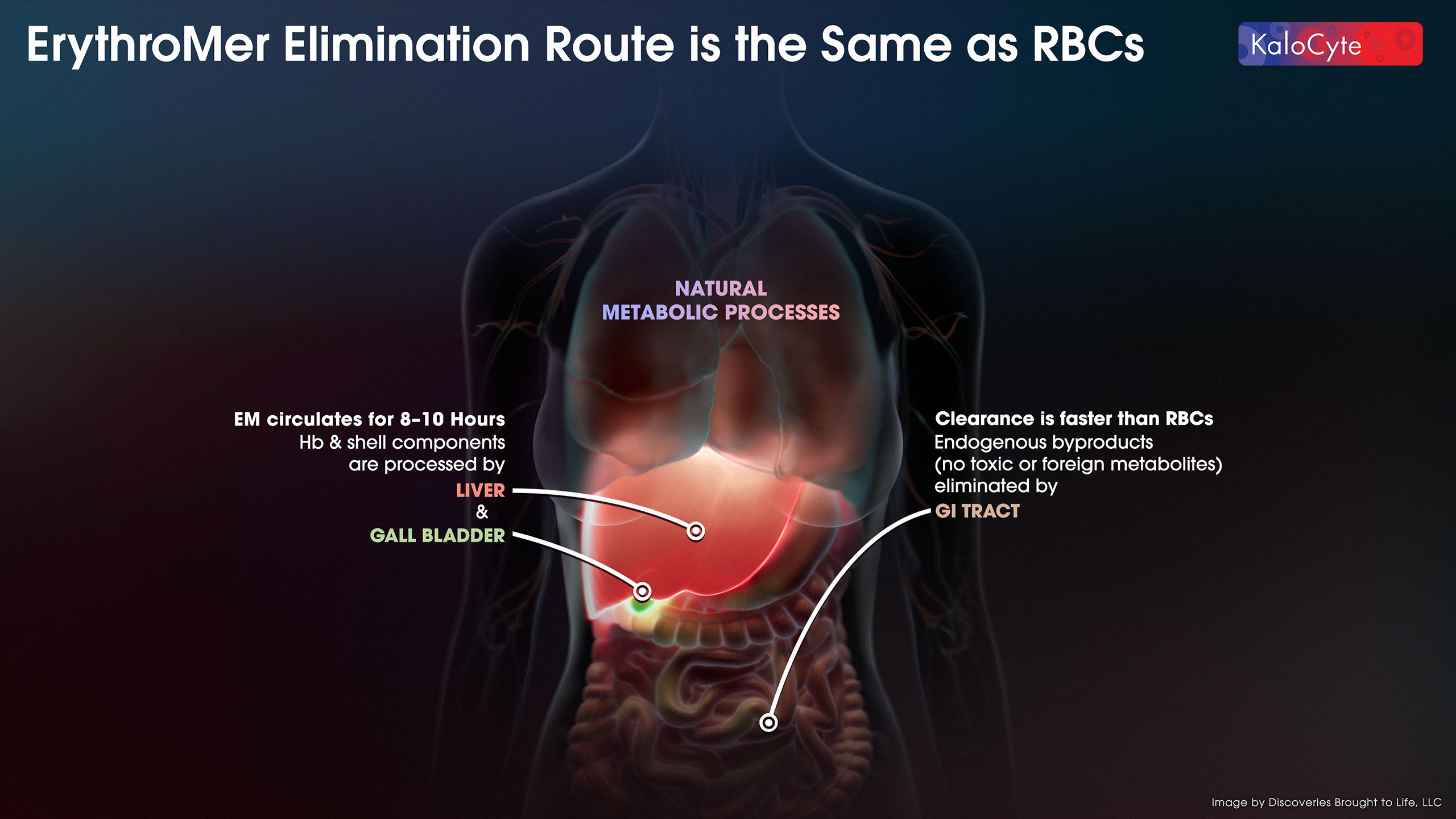
Based on our current preclinical studies, the current ErythroMer™ design in development will be effective for about 8 – 10 hours between doses and cleared just like native cells, with all natural byproducts. Hb and shell components are processed by the liver and gall bladder. Endogenous byproducts are eliminated by the GI tract.
The polar head groups of phospholipid components in the EM shell are presumed to be converted by phospholipases into endogenous phospholipids, such as phosphatidyl choline. The amino acid in the proprietary KC1003 molecule may likely be converted to agmatine by arginases. Metabolism of PEG is well defined, producing end products that enter the TCA cycle (a.k.a. Krebs Cycle) or are incorporated during gluconeogenesis.
COMPETITION
In terms of competition, the standard of care is blood – a type-specific and perishable liquid that requires refrigeration, cold-chain, and use within about a month, so there have been many attempts to develop a substitute with more ideal features that improve upon these limitations. Attempts to date have failed due to two main reasons:
-
- Poor oxygen delivery, and
- Safety and efficacy issues that ended FDA trials.
ErythroMer™ was specifically designed to fix these issues, and we have a growing set of preclinical data to support its future use in humans. In studies to date, we are demonstrating safety and efficacy in preclinical studies – in vivo, ex vivo, and in vitro. None of the first-generation HBOC attempts are viable for use in trauma, and other technologies in development either lack ideal features or are far behind us in development.
Imagine if every first responder and hospital had shelf-stable blood stocked for use on any patient needing an emergency transfusion. At a price approximating the fully-loaded cost of a unit of blood, the market potential is $7 billion in the US alone.
ErythroMer™ – a lifeline when blood is not available.
QUICK LINKS
ABOUT US
KaloCyte was founded by a distinguished team of researchers in physiology, bioengineering, and trauma care and is poised to deliver ErythroMer, a dried, bio-inspired artificial red blood cell, to market. ErythroMer is envisioned for use when stored red blood cells are unavailable, undesirable or in short supply. KaloCyte is supported by nearly $20M in federal grants and investor funding.